AbbVie Careers

AbbVie: Still Positioned To Deliver Alpha Over The Next Decade
AbbVie’s acquisition of Allergan will create a new company churning out $20 billion in free cash flow a year, and that makes it worth buying, UBS’ Navin Jacob said Thursday. Because scale-up and technology transfer of the lyophilization process is challenging, it is important to develop a comprehensive understanding of critical lyophilization characteristics early on. Partnering with an experienced contract development and manufacturing organization (CDMO) for lyophilization can shorten the drug development process and expedite time to market. By choosing AbbVie CMO for development and manufacture of your mAb, you’re not only selecting a CMO, you’re selecting a pharmaceutical partner. In making this choice, you will benefit from the scientific expertise of a company with a proven track record in biopharmaceutical process development, manufacturing and commercialization.
News AbbVie Inc.ABBV
Here’s a look. Devised as the research arm of Abbott and based in Chicago, http://salalah-mills.com/2019/10/01/dezodorant-garnier-neo/ became a major pharmaceutical company in its own right in 2013. The diminished Abbott now retains the non-research interests – everything from baby formula to sports nutrition to heart stents. As for AbbVie and its separation from its progenitor, management claimed that the move gave investors a chance to objectively value two businesses that were heading in disparate directions.
Trump Rule Requiring Drug Prices in TV Ads Blocked
The notes are being offered in conjunction with an exchange offer for the outstanding senior unsecured notes of subsidiaries of Allergan plc including Allergan, Inc., Allergan Sales, LLC, Allergan Funding SCS and Allergan Finance, LLC (collectively “Allergan”). AbbVie is an American publicly traded biopharmaceutical company founded in 2013. It originated as a spin-off of Abbott Laboratories. (Crain’s) — Abbott Laboratories has named its new spinoff AbbVie as it prepares to launch the company by yearend.
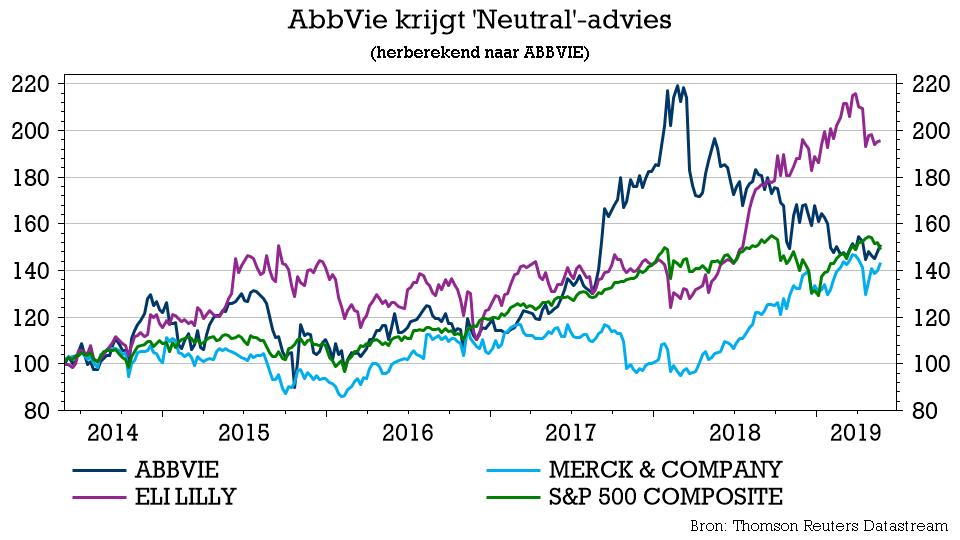
AbbVie Inc. discovers, develops, manufactures, and sells pharmaceutical products in the United States, Japan, Germany, Canada, Italy, Spain, the Netherlands, the United Kingdom, Brazil, and internationally. The company offers HUMIRA, a therapy administered as an injection for autoimmune and intestinal Behçet’s diseases; IMBRUVICA to treat adult patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma, waldenström’s macroglobulinemia, marginal zone lymphoma, and chronic graft versus host disease; VENCLEXTA, a BCL-2 inhibitor used to treat adults with CLL or SLL; VIEKIRA PAK, an interferon-free therapy to treat adults with genotype 1 chronic hepatitis C virus (HCV); TECHNIVIE to treat adults with genotype 4 HCV infection; and MAVYRET to treat patients with chronic HCV genotype 1-6 infection. It also provides KALETRA, an anti-human immunodeficiency virus (HIV)-1 medicine used with other anti-HIV-1 medications to maintain viral suppression in HIV-1 patients; NORVIR, a protease inhibitor indicated in combination with other antiretroviral agents to treat HIV-1; and SYNAGIS to prevent respiratory syncytial virus infection at-risk infants.
Cynical investors retorted that Abbott’s board of directors constructed http://www.jewishcare.org.za/kriptovaljuty/5-kriptovaljut-kurs-kotoryh-ne-perestaet-rasti as a repository in which to dump some of its imminently expiring patents. Operating from multiple locations worldwide, AbbVie Biologic’s center of excellence is conveniently located just outside of Boston in Worcester, Massachusetts. This primary bioresearch and manufacturing site is complemented by manufacturing centers in Puerto Rico and Singapore, making up some of the most technologically advanced facilities in the world. Our sites offer global pharmaceutical companies a high degree of flexibility, scale, and wide-ranging capabilities to support every stage of mAb development and manufacture.
- Pharmaceutical company Abbott Laboratories (ABT) now has a considerably smaller market capitalization than does spinoff AbbVie Inc. (ABBV), which is now worth $140.3 billion as of July 27, 2018.
- Reata Pharmaceuticals, Inc. announced the reacquisition of development, manufacturing and commercialization rights concerning its proprietary Nrf2 activator product platform originally licensed to AbbVie, Inc.
- AbbVie is a global, research and development-based biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions.
For regulatory reasons we have to ensure you are aware of the appropriate regulations for the country which you are in. To allow you to view details relating to the Offer, you have to read the following and then press “I agree”.
NORTH CHICAGO, Ill., Oct. 29, 2019 /PRNewswire/ — https://www.pinscher-miniatura.com/nzd-cad-torgovlja-s-maxitrade-com/ (ABBV), a research-based global biopharmaceutical company, today announced the launch of Let Me Be Clear, an empowerment platform for people living with psoriasis. In October, Abbott surprised investors and analysts when executives announced they would spin off the company’s branded drug business, which includes the blockbuster arthritis drug Humira. Reata Pharmaceuticals, Inc. announced the reacquisition of development, manufacturing and commercialization rights concerning its proprietary Nrf2 activator product platform originally licensed to AbbVie, Inc. BioMed X announced the successful completion of their first research collaboration project with AbbVie in the field of tau-mediated neurodegeneration in Alzheimer’s disease.
The company was incorporated in 2012 and is headquartered in North Chicago, Illinois. Cramer said Wall Street is only now waking up to just how transformative Allergan will be to AbbVie, and now’s the time to take advantage as the combined company will have both growth and an excellent dividend yield.
In particular, you certify that you will not forward or transmit the Information either in whole or in part to any person in any jurisdiction where such distribution may be restricted by applicable law or regulation. Failure to comply with any such restrictions may constitute a violation of the laws and/or regulations of any such jurisdiction. CONFIRMATION OF UNDERSTANDING AND ACCEPTANCE OF THIS NOTICE By clicking on “I agree” below, you confirm that you have read, understood and agreed to be bound by the terms of the notice set out above and that you are not in, or a resident of, any jurisdiction where to download or view the Information would constitute a breach of securities law or regulation in that jurisdiction. If you click “I disagree” below, we will be unable to provide you with access to the Information and you will be redirected to AbbVie’s homepage.
Recommended Offer for Allergan plc (“Allergan”) by AbbVie Inc. (“AbbVie”) (the “Acquisition”) by means of a scheme of arrangement under Irish law (the “Scheme”). You are attempting to enter the section of this website that is designated for the publication of documents and information in connection with the offer by AbbVie for Allergan announced on June 25, 2019 (the “Offer”). ACCESS TO THIS SECTION OF THE WEBSITE MAY BE RESTRICTED UNDER SECURITIES LAWS IN CERTAIN JURISDICTIONS. THIS NOTICE REQUIRES YOU TO CONFIRM CERTAIN MATTERS (INCLUDING THAT YOU ARE NOT RESIDENT IN SUCH A JURISDICTION), BEFORE YOU MAY OBTAIN ACCESS TO THE INFORMATION. THE INFORMATION IS NOT DIRECTED AT, AND IS NOT INTENDED TO BE ACCESSIBLE BY, PERSONS RESIDENT IN ANY JURISDICTION WHERE TO DO SO WOULD CONSTITUTE A VIOLATION OF THE RELEVANT LAWS OF THAT JURISDICTION. IF YOU ARE NOT PERMITTED TO VIEW THE INFORMATION, OR VIEWING THE INFORMATION WOULD RESULT IN A BREACH OF THE ABOVE, OR YOU ARE IN ANY DOUBT AS TO WHETHER YOU ARE PERMITTED TO VIEW THE INFORMATION, PLEASE EXIT THIS WEBPAGE. THIS SECTION OF THE WEBSITE CONTAINS ANNOUNCEMENTS, DOCUMENTS AND INFORMATION (TOGETHER THE “INFORMATION”) RELATING TO THE OFFER IN COMPLIANCE WITH THE IRISH TAKEOVER PANEL ACT, 1997, TAKEOVER RULES 2013 (THE “IRISH TAKEOVER RULES”). THE INFORMATION IS BEING MADE AVAILABLE IN GOOD FAITH AND FOR INFORMATION PURPOSES ONLY, AND ITS AVAILABILITY IS SUBJECT TO THE TERMS AND CONDITIONS SET OUT BELOW. THE INFORMATION IS NOT INTENDED TO, AND DOES NOT, CONSTITUTE OR FORM ANY PART OF AN OFFER TO SELL OR OTHERWISE DISPOSE OF OR AN INVITATION OR THE SOLICITATION OF AN OFFER TO PURCHASE OR OTHERWISE ACQUIRE ANY SECURITIES, OR THE SOLICITATION OF A VOTE OR APPROVAL PURSUANT TO THE INFORMATION OR OTHERWISE. ANY PERSON SEEKING ACCESS TO THIS SECTION OF ABBVIE’S WEBSITE REPRESENTS AND WARRANTS TO ABBVIE THAT THEY ARE DOING SO FOR INFORMATION PURPOSES ONLY. BASIS OF ACCESS TO INFORMATION Please read this notice carefully before clicking “I agree” or “I disagree” below.
Moreover, our total commitment to treating your program as if it were our own ensures strong collaboration and ultimate success all the way from innovation to commercialization. One of the most challenging aspects in developing an HME process for manufacturing an ASD is to achieve the balance between obtaining a uniform ASD by providing sufficient mixing and dispersing while minimizing degradation of the drug and/or polymer. A twin screw extruder design is favorable for pharmaceutical applications due to its superior mixing capability and shorter material residence time. Extrusion is an integrated unit operation consisting of different functional zones (e.g. conveying, mixing, melting, degassing, shaping, etc.).
The interplay between material properties and energy applied in different zones throughout the extruder is fairly complicated and relevant controls need to be identified and implemented. Individual process parameters, including feed rate, screw speed, barrel temperature profile, and vacuum can be adjusted independently to meet product quality requirements. AbbVie is a global, research and development-based biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions.
The Information may not be downloaded or accessed by any person from or in any jurisdiction where it would or may constitute a breach of any applicable laws or regulations. If you are not permitted to view or download the Information on the website, or viewing or downloading the Information would result in a breach of the above, or you are http://stage3.athens.kiev.ua/2019/10/01/kurs-golem/ in any doubt as to whether you are permitted to view or download the Information, please exit this webpage by clicking on the “I disagree” box below. By clicking on the “I agree” box below, you certify that you will not forward, transmit, show or distribute (by any means including by electronic transmission) the Information to any person.




